Researchers from the University of Alabama, Birmingham report on the use of the SynVivo platform for the development of a human airway-on-a chip model. Combined with novel micro-optical coherence tomography (µOCT), this model enables non-invasive quantitative imaging of ciliary movement. The imaging includes beat frequency and mucociliary transport.
“The advantage of this microfluidics device lies in the formation of a complete lumen for both the airway epithelium and the adjacent endothelium. It is a step forward in the development of a model that recapitulates both the cellular differentiation and organization into tubular structures, similar to the small airways and microvasculature”, said Dr. Jennifer Guimbellot, pediatric pulmonologist and assistant professor, UAB School of medicine.
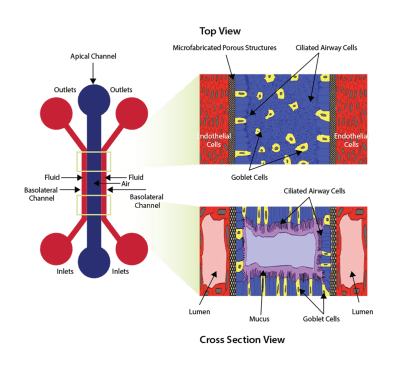
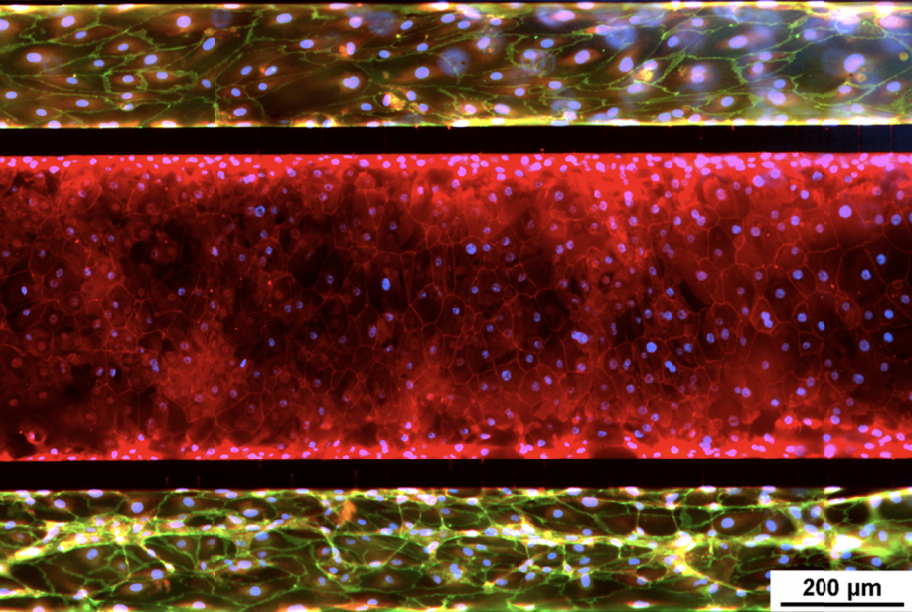
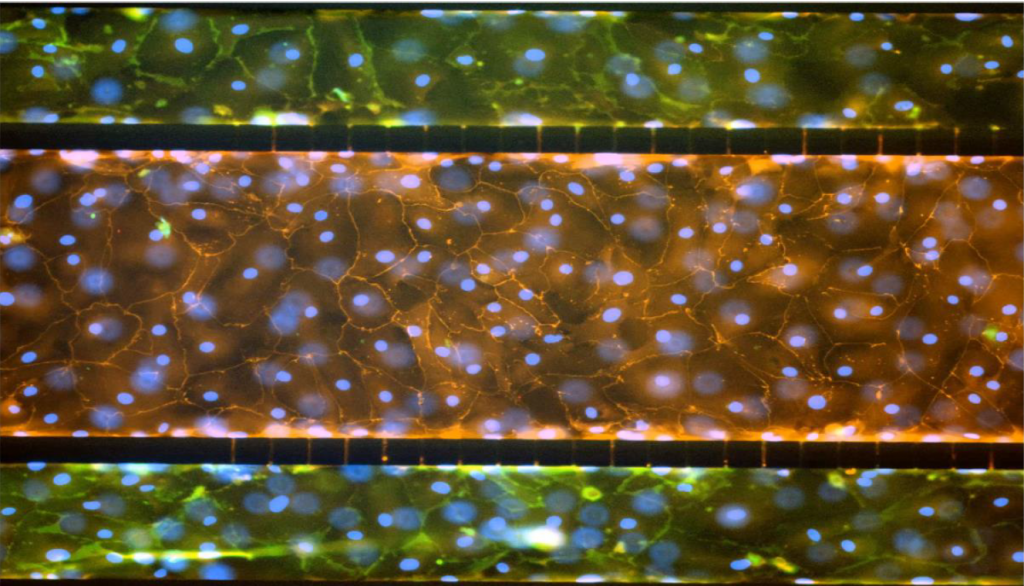
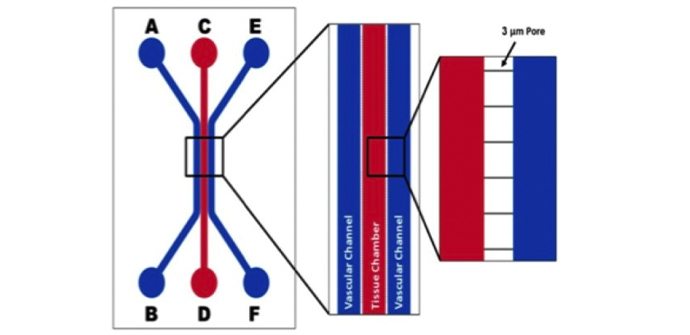
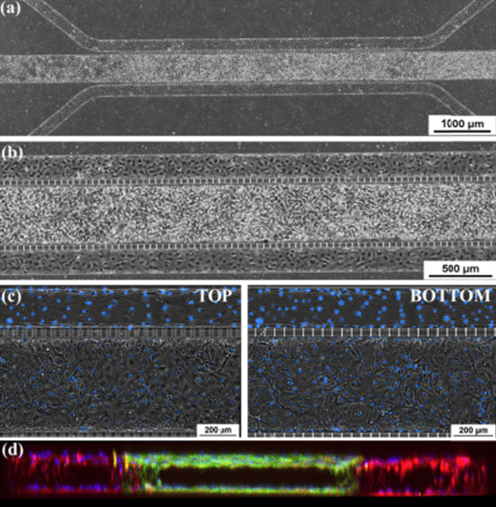
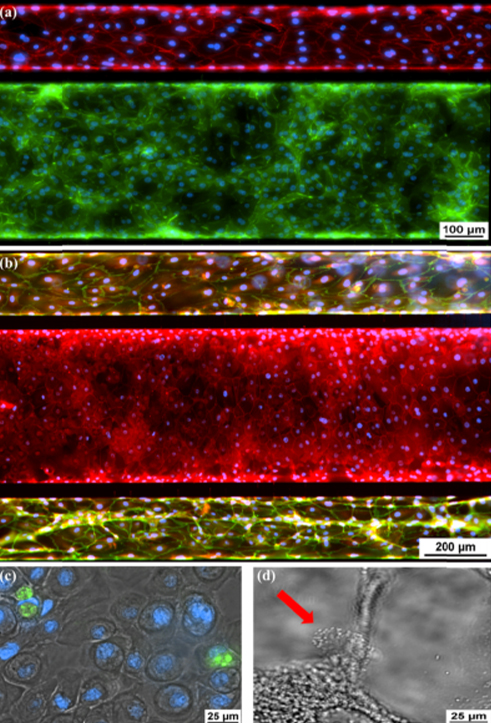
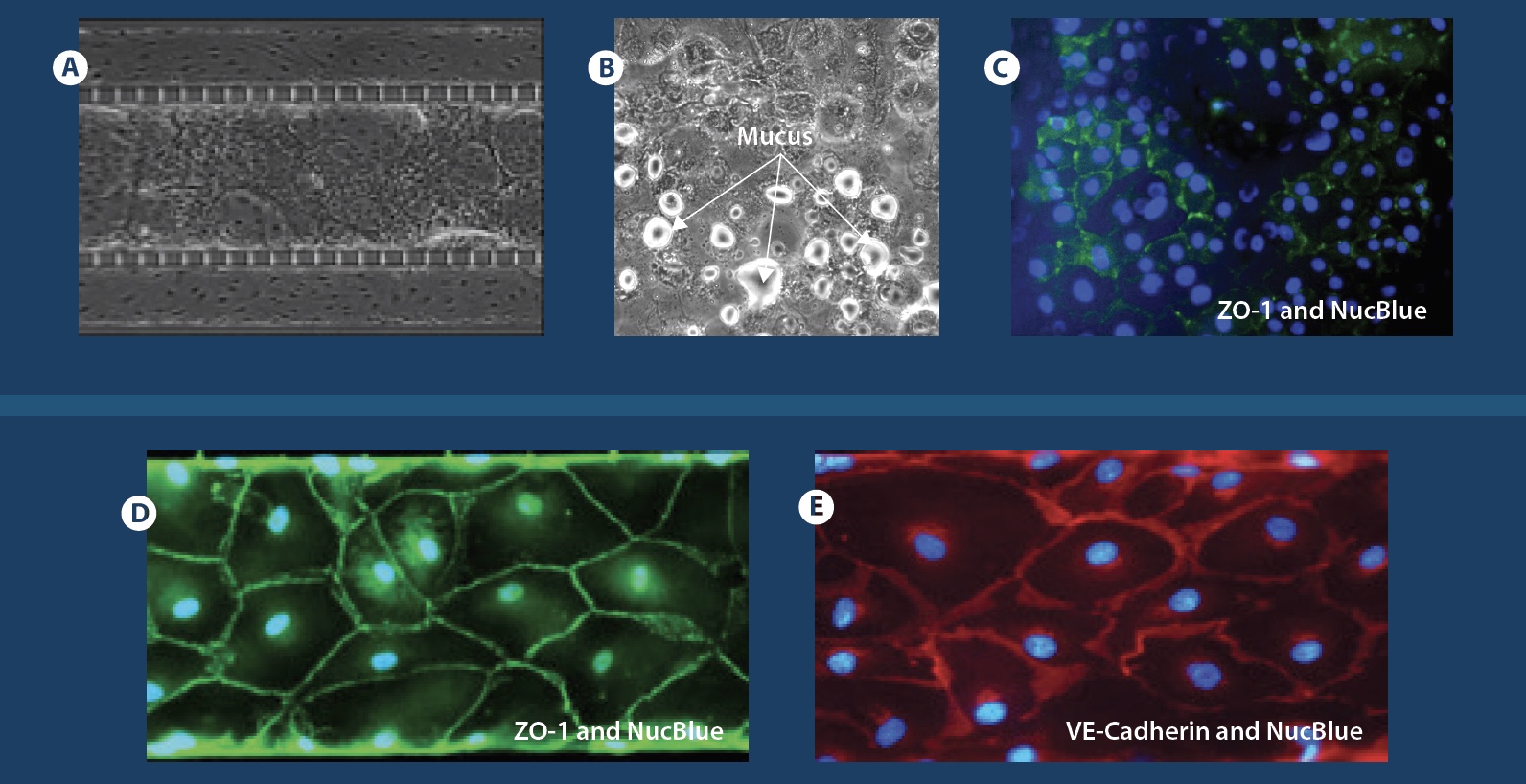
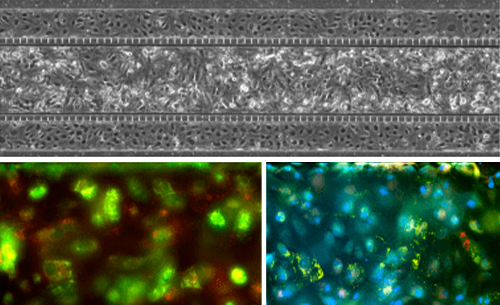
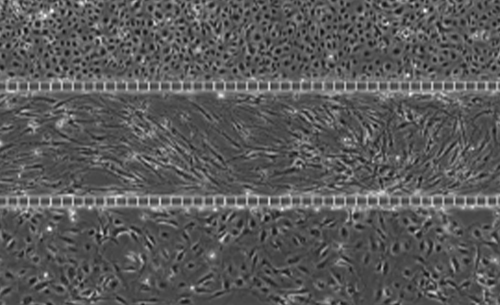
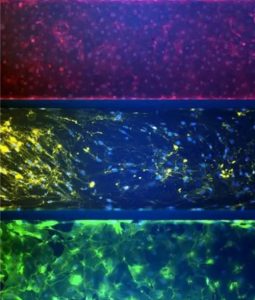
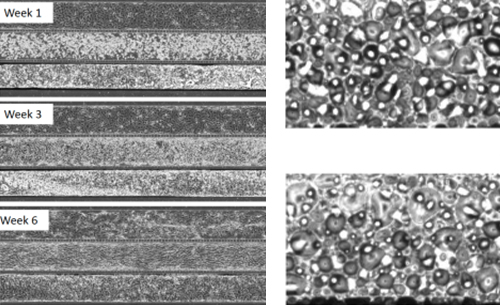
The airway model was developed with a co-culture of primary epithelial cells and endothelial cells across an Air Liquid Interface (ALI). It was created using a customized SynVivo microfluidic chip enabling real-time quantitative imaging. The functionality of the developed airway-on-a-chip model was demonstrated by monitoring active cilia, mucus-producing cells, and biomarkers of cellular function under physiological conditions.
Validation From Researchers
According to Dr. Steven Rowe, Professor of Medicine and Director of the Gregory Fleming James Cystic Fibrosis Research Center: “Developing new tools that appropriately model the intact mucociliary transport apparatus of humans is a major priority, and has implications for biological research of airway diseases including cystic fibrosis”. The developed airway model represents a new approach to personalized medicine and as a predictive tool for pharmaceutical development.
Co-cultured microfluidic model of the airway optimized for microscopy and micro-optical coherence tomography imaging
Authors: Zhongyu Liu, Stephen Mackay, Dylan M. Gordon, Justin D. Anderson, Dustin W. Haithcock, Charles J. Garson, Guillermo J. Tearney, George M. Solomon, Kapil Pant, Balabhaskar Prabhakarpandian, Steven M. Rowe, and Jennifer S. Guimbellot.
Biomedical Optics Express Vol. 10, Issue 10, pp. 5414-5430 (2019).